
Welcome to Leader in Water- & Wastewater Solution
Welcome to Leader in Water- & Wastewater Solution
Hydrogen production from electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) by using an electric current. This hydrogen produced from the electrolytic process is used in industrial applications today.
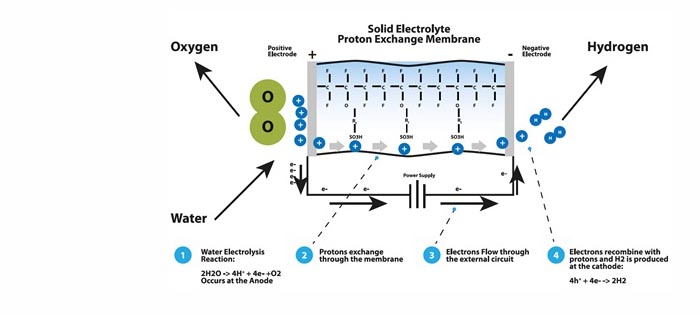
An electrical power source is connected to two electrodes, or two plates, (typically made from some inert metal such as platinum or stainless steel) which are placed in the water. Hydrogen is produced at the cathode (the negatively charged electrode, where electrons are pumped into the water), and oxygen is produced at the anode (the positively charged electrode). The generated amount of hydrogen is twice the amount of oxygen, and both are proportional to the total electrical charge being sent through the water. Electrolysis cells are characterized by their electrolyte type. There are two types of low temperature electrolysis: alkaline and proton exchange membrane (PEM). Alkaline electrolysis utilizes a liquid electrolyte consisting of highly concentrated potassium hydroxide (KOH). PEM electrolysis is based on the use of a solid conducting polymer that conducts ions when hydrated with water.
Proton Exchange Membrane electrolyzers are sold worldwide and are used in a variety of industrial and laboratory applications, including heat treating, printed circuit board manufacturing, cooling of power plant turbine generator windings, weather balloon filling, and gas chromatography. These electrolyzers have demonstrated high reliability in a wide range of environments and duty cycles.